Peptide News !!!
- Home
- Peptide News !!!
NEWS FROM THE PEPTIDE WORLD
Updates about all new developments in peptide science, including new methods, products, instrumentation and peptide based drugs
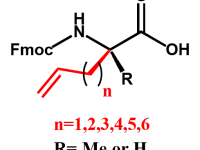



THE Thalidomide Paradox
Brief Introduction
Thalidomide was a drug responsible for limb malformations in the late 1950s when it was prescribed for pregnant women to relieve symptoms of morning sickness. Although thalidomide was banned in the mid-1960s worldwide, it recovered clinical interest because of its unique pharmacological action against leprosy, HIV and cancer. Now, it is on the market with the name THALOMID® for the treatment of patients with multiple myeloma or severe erythema nodosum leprosum.
The S and R
thalidomide
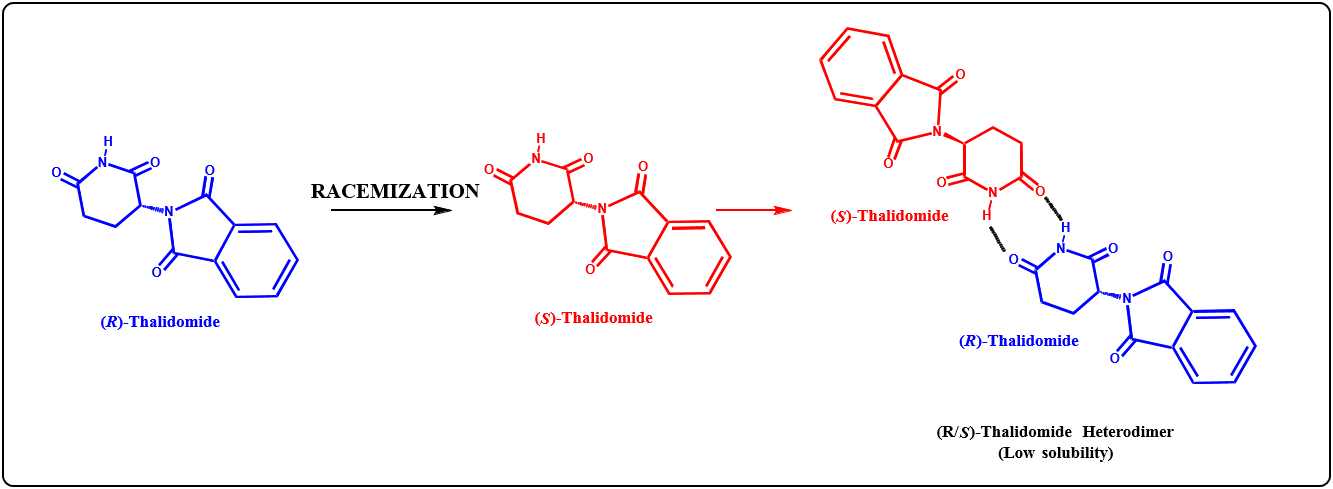
In 1979, Blaschke et al. discovered that the S and R thalidomide enantiomers display different biological properties. Only the (S)-enantiomer
is responsible for the teratogenic side effects, while no teratogenicity was
observed for the (R)-isomer in animal experiments. However, the (R)-thalidomide can undergo considerable racemization after incubation in buffer solution (τ1/2 = 12 h) as well as in serum (τ1/2 = 1 h). According to these results, the teratogenic side effects should be observed because the (S)-isomer is now present, but Blaschke did not observe these side effects.
In 2010, Handa and co-workers carried out a landmark
biological study on thalidomide by identifying cereblon (CRBN) as a
thalidomide-binding protein. They found that thalidomide induces its teratogenic effects by binding to CRBN in zebrafish and chicks. The publication of this report opened up a new era for thalidomide in science.
The biochemical studies were carried out on deuterium-substituted enantiomers of thalidomide to suppress any enantiomeric interconversion. The results revealed that the (S)-enantiomer of thalidomide displayed a 10-fold
stronger binding to CRBN and inhibition of self-ubiquitination, compared with the (R)-isomer. It means that the results reported by Blaschke are correct and that the teratogenic effects of thalidomide are exclusively induced by the (S)-enantiomer.
Thalidomide Paradox
However, one question remains regarding the clear biological
differences between the thalidomide enantiomers reported in 1979, despite the observed racemization of thalidomide. Why do animal experiments that use (R)-thalidomide not display teratogenicity if the (R)-isomer readily racemizes in vivo? This discrepancy is known as the “thalidomide paradox.”
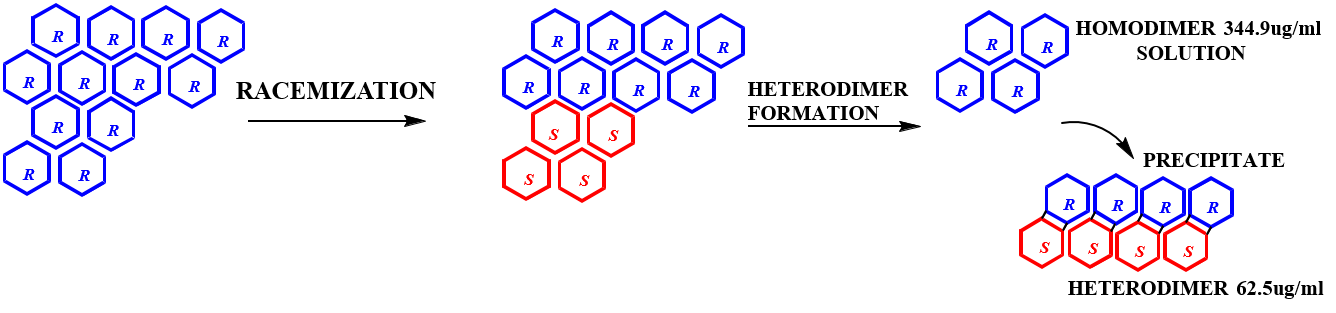
A Good Answer
After carefully conducting studies on pure isomers and racemic samples, Shibata et al concluded that the (R)-thalidomide and (S)-thalidomide are very soluble in water (homodimers = 344.9 ug/ml). However, after racemization, the (R)and (S) thalidomide enantiomers will form a less soluble heterodimer (R-S)-thalidomide (heterodimer = 62.5 ug/ml). This heterodimer will precipitate, thus avoiding the biological effects. The (R-S)-thalidomide heterodimer has stronger hydrogen bonds (2.895Ǻ) than the R-R and S-S thalidomide homodimers (2.912Ǻ). It is also possible that the (S)-isomer that is newly formed through racemization of the (R)-isomer will not bind to CRBN because it is blocked by the formation of the strong heterodimer.
References:
Tokunaga, E.,Yamamoto, T., Ito, E. et al. Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers Sci. Rep. 8, 17131 (2018).=
Blaschke G, Kraft HP, Fickentscher K, Köhler F.
Chromatographische Racemattrennung von Thalidomid und teratogene Wirkung der Enantiomere [Chromatographic separation of racemic thalidomide and teratogenic
activity of its enantiomers (author’s transl)]. Arzneimittelforschung. 29,10:1640-2. (1979) German. PMID: 583234.
Ito, T. et al. Identification of a Primary Target of
Thalidomide Teratogenicity. Science 327, 1345–1350 (2010).
Chamberlain, P. P. et al. Structure of the human
Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 21, 803–809 (2014).
Peptides
ABOUT CELL PENETRATING PEPTIDES…
Towards understanding cell penetration by stapled peptides
Chu, Q.; Moellering, R.E.; Hilinski, G.J.; Kim,Y.W; Grossmann, T.N.; Yeh, J.T.H; Verdine G. L.
Med. Chem. Commun., 2015, 6,111
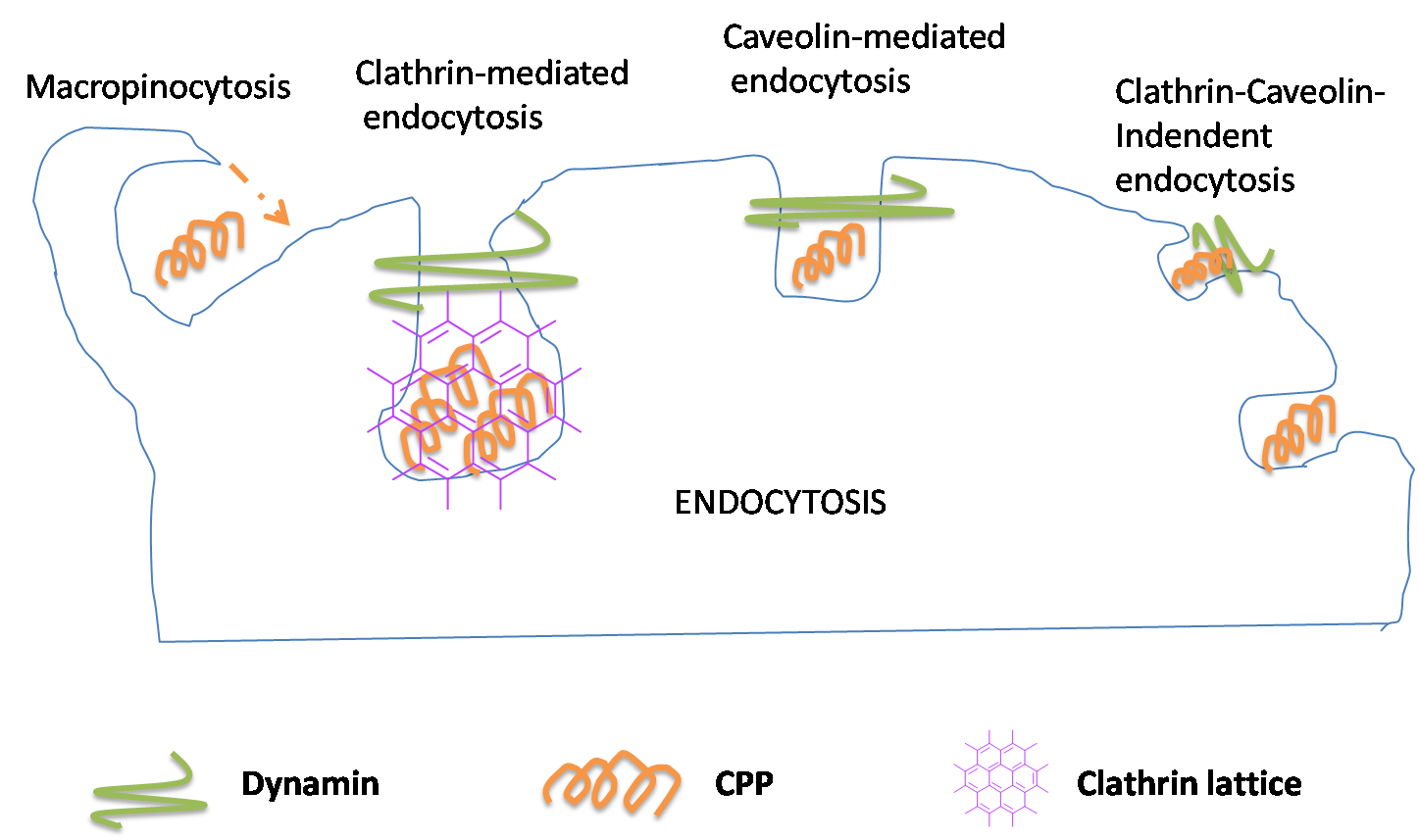
“A direct comparison with several well-known CPPs has revealed that stapled peptides, including some stapled versions of the CPPs, exhibit more robust cell penetration.”
Cell-Penetrating Peptides Derived from Animal Venoms and Toxins
An increasing number of studies have reported on the applicability of venom peptides in the diagnosis and prospective treatment of diseased processes that are dependent on selective and specific cell membrane interaction and/or receptor binding, translocation across the cell membrane (cell penetration), intracellular trafficking and subcellular localization to exert their effect.
Rádis-Baptista, G.
Cell-Penetrating Peptides Derived from Animal Venoms and Toxins.
Toxins 2021, 13, 147.

fall in love with our services
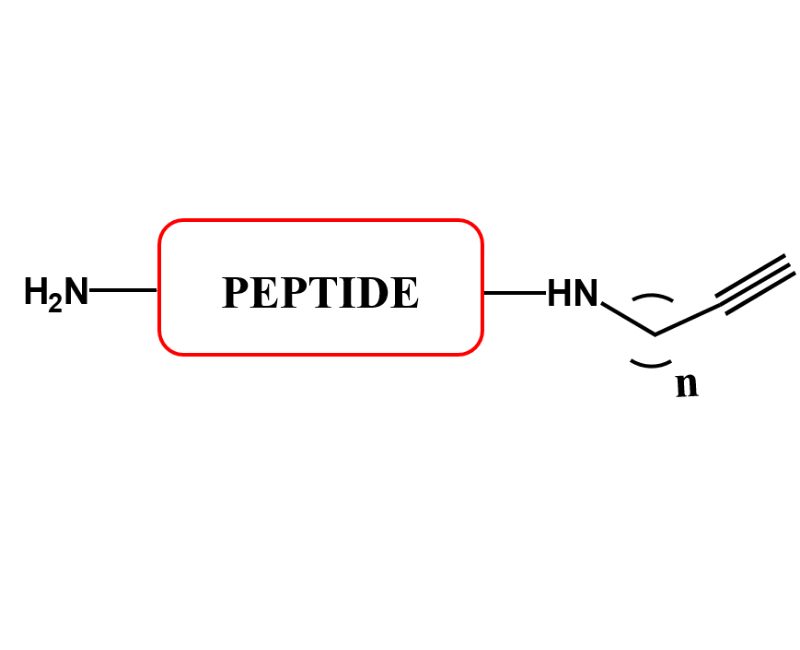
C-TERMINAL PEPTIDES
IN ADDITION TO THE ACID AND AMIDE C-TERMINI, WE CAN ALSO PROVIDE ALCOHOL, n-ALKYL AMIDES AND MORE
N-TERMINAL MODIFICATIONS
WE CAN PROVIDE BIOTINYLATED, PEPTIDES WITH ANY KIND OF SPACER. FLUORESCEIN, FITC AND MORE DYES. fATTY ACIDS LIKE MIRISTIC, PALMITIC AND OLEIC CAN ALSO BE INCORPORATED AL THE n-TERMINUS.

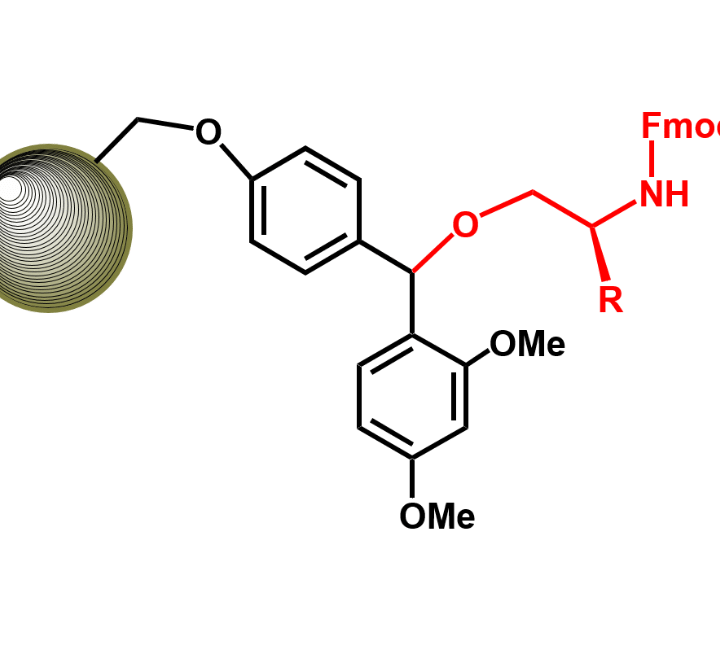
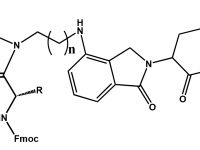
PROTAC PEPTIDE CONJUGATES
The PROTAC system for selective protein destruction. They are constructs made up of two molecules that are connected by a linker. The activity of PROTACS is dependent on these connected molecules. One end binds to the protein of interest (POI), while the other binds to an E3 ligase. PROTACs hijack the ubiquitin-proteosome system (UPS) to induce ubiquitination and degradation of the POI by bringing it in close proximity to the E3 ligase.
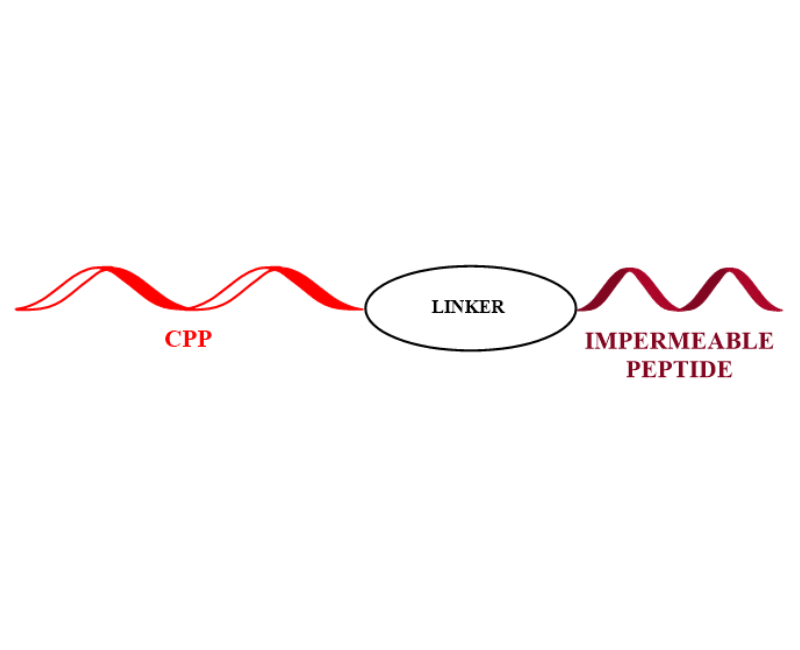
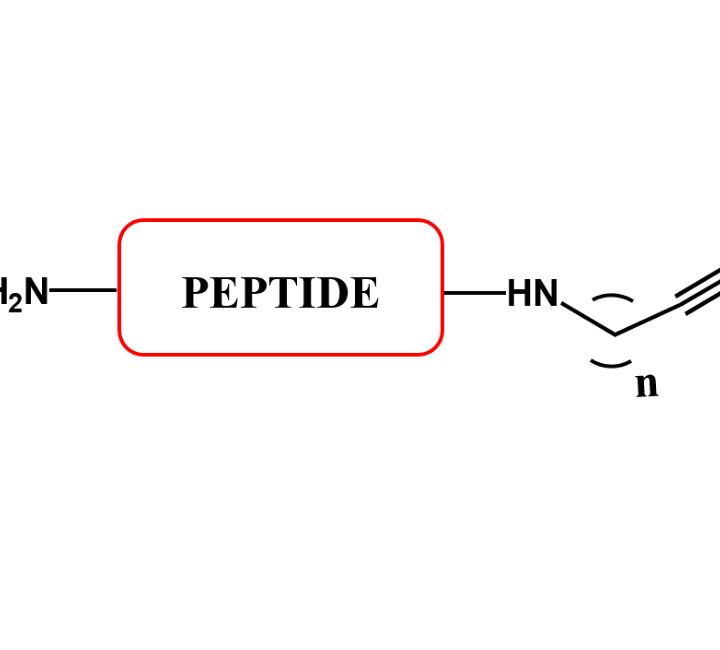
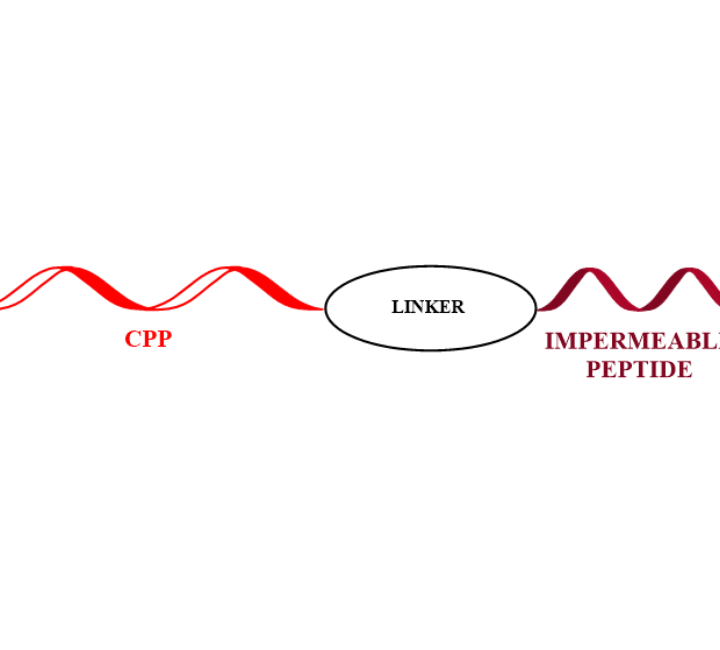
CPP PEPTIDE CONJUGATES
Cell penetrating peptides have been successfully used for delivering a wide variety of therapeutic molecules into various types of cells for the treatment of multiple diseases. These peptides can internalise in many type of cells.
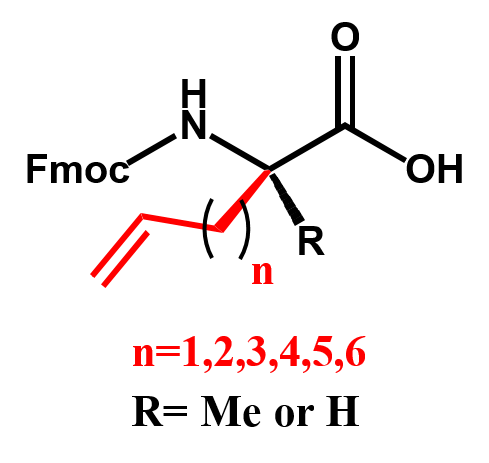
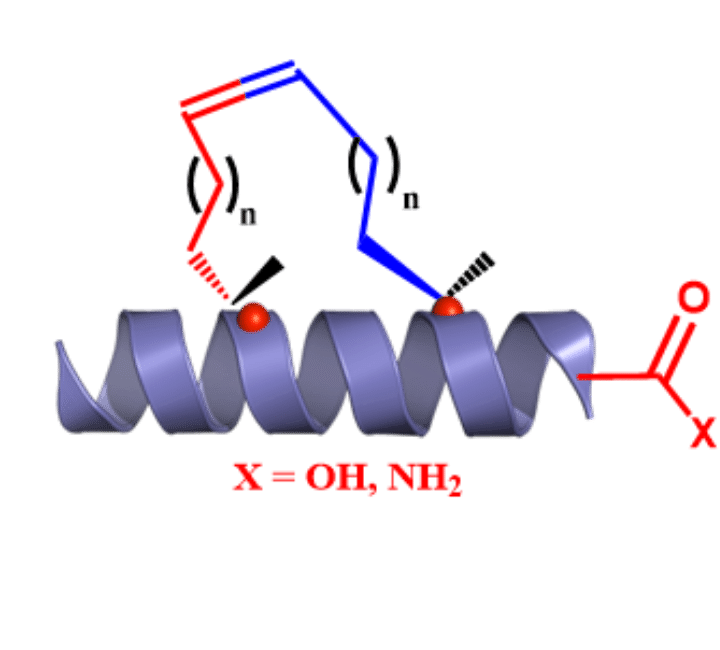
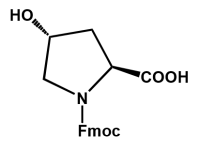
SPECIAL AMINO ACIDS
We offer competitive prices on the Fmoc-alkenyl alanines used for the synthesis of all hydrocarbon Stapled α−helical peptides (AHSP). AHSP have been used to disrupt the interaction between p53 and MDM2 and MDMX which are overexpressed in some types of cancers restoring the p53-dependent cell-cycle arrest and apoptosis in several class of tumors.

GIVE US A CALL
+1 657 242-0307 or send us an e-mail: fernando@delivertides.com
LIFE IS SHORT, MAKE PEPTIDES !!!
We can help you...
Image Source: WOCinTechChat, Icon Finder
JUST GIVE US YOUR PEPTIDE SEQUENCE AND WE CAN MODIFY IT TO YOUR NEEDS
We will change your peptide at the C-termini. Peptide alcohols and N-alkyl amides are more stable and more hydrophobic.



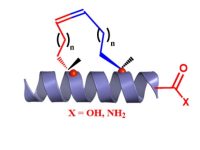
Experience In stapled peptides
All hydrocarbon stapled and stitched peptides
Delivertides can synthesize i,i+3; i,i+4; and i,i+7 double stapled and stitched peptides for your research needs.
All hydrocarbon stapled α-helical peptides (AHSP) have emerged as a promising new modality to address a wide range of therapeutic targets.
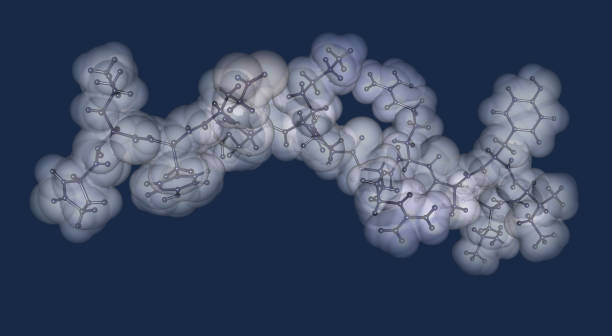

your necessities
our priorities
We can work together in order to increase permeability of your your active peptide.
We will design your new peptides taking your benchmark sequence sharing our experience in peptide SAR