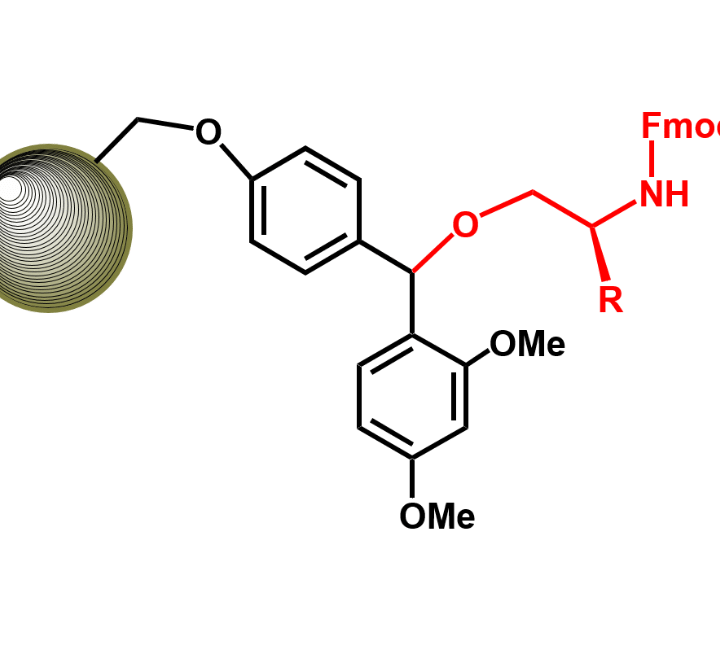
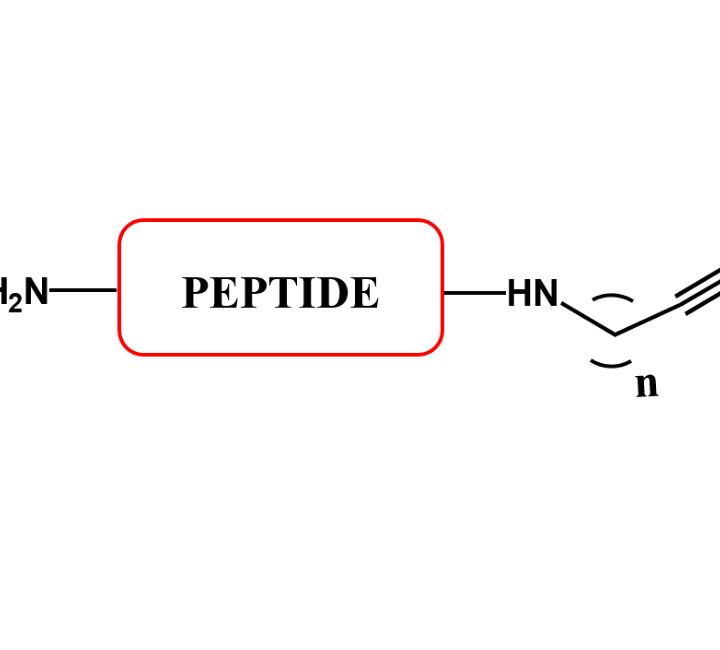
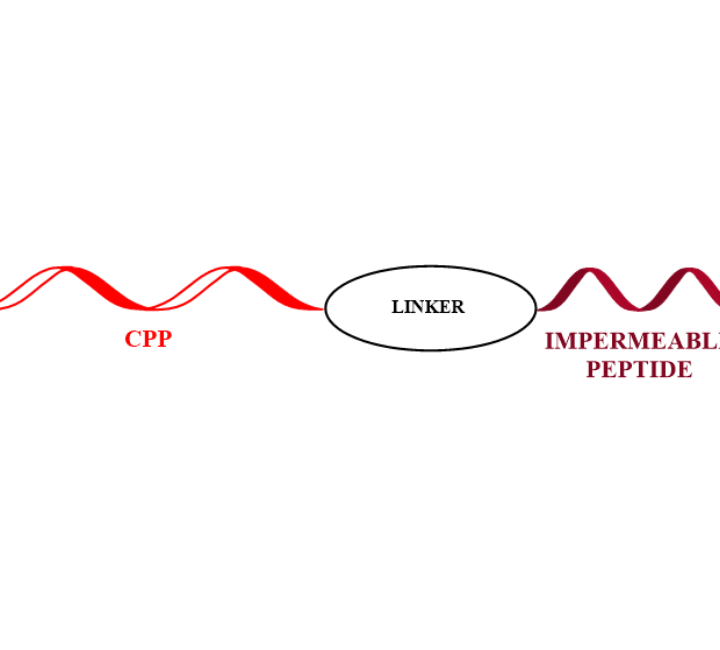
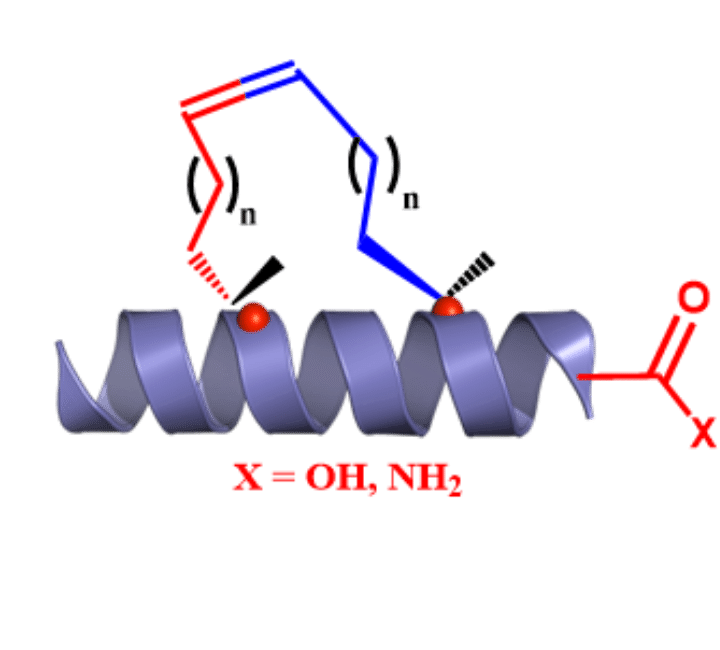
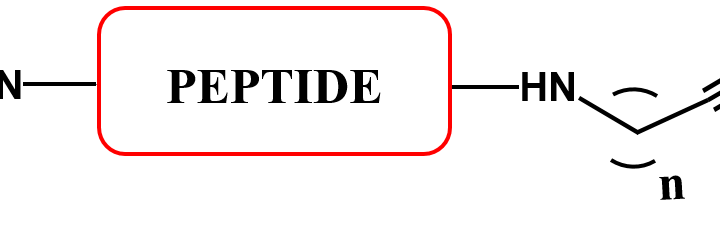
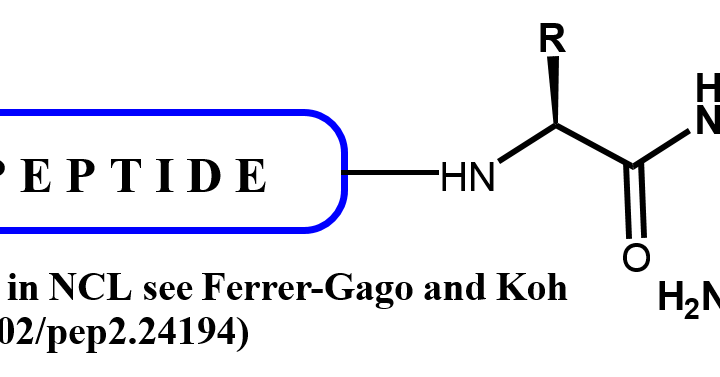
C-Terminal Peptide Modifications
- Home
- C-Terminal Peptide Modifications
CPP thioacids (CPP-SH) as active peptides
A CPP-SH has the following general formula:
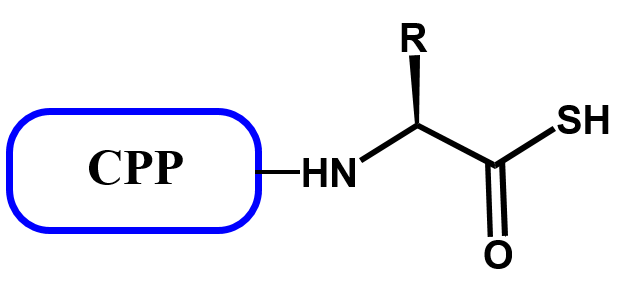
Peptide thioacids are known in the literature including penetratin thioacid (Ac-RQIKIWFNRRMKWKKF-SH)
This method is based on the reaction of C-terminal peptidyl thioacid with an electrondeficient
N-terminal sulfonamide to yield a native amide bond. The mechanism of this reaction, involves nucleophilic aromatic substitution by the thiocarboxylate on the electrondeficient
sulfonamide to give a highly reactive thioester, after loss of sulfur dioxide, an amine leading ultimately to the amide product.
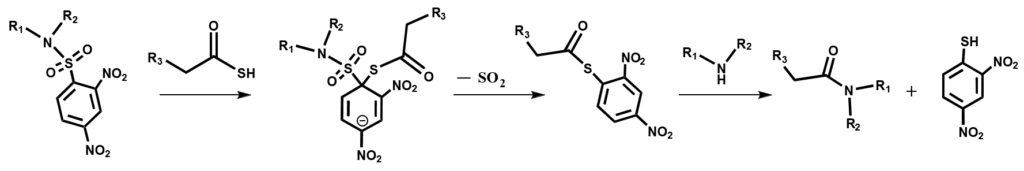
As an example, Penetratin-SH can be ligated to an additional dinitrosulfonamide N-terminal peptide (DNS-N-terminal peptide).
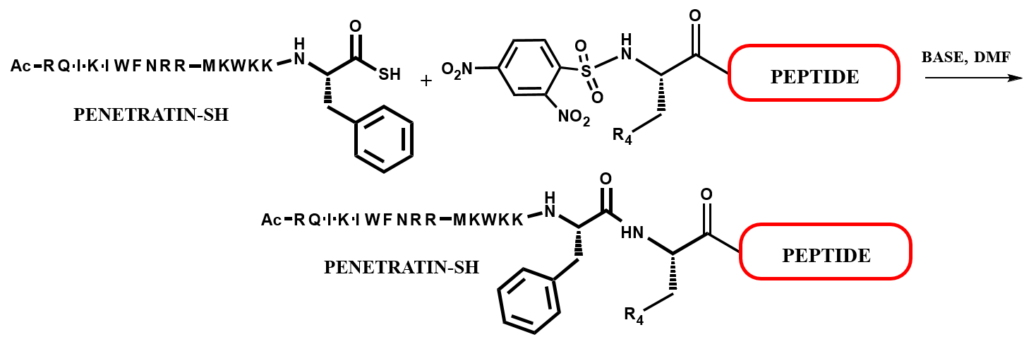
This application allows the incorporation of the same CPP to a different set of peptides without the necessity of performing a linear synthesis of each CPP peptide conjugate.
An additional advantage of this approach is that no additional linkers like maleimide an other are needed. An amide bond is the final link between the CPP and the additional peptide.